
Corrosive influence - The dangers of galvanic corrosion for pipelines
Geir Otto Amundsen on Case Studies · Apr 22, 2020
Prevention is always better than a cure. Avoiding issues in the first place, rather than attending to them once they’ve occurred, is plain common sense. So why do so many industrial facilities install piping networks where gaskets and flanges are made from different metals, opening their doors to one of industry’s most unwelcome (and costly) guests – galvanic corrosion?
Corrosion isn’t just a technical, operative or maintenance issue – it’s a business-critical challenge.
The cost of corrosion
The degradation of metals within industry environments is dangerous (leading to fugitive emissions and accidents), inefficient (with the capacity to halt production) and often very, very costly, in terms of both maintenance and facility downtime.
In fact, a study by NACE International, the worldwide authority on corrosion, revealed that the global cost of the issue to industry is a staggering USD 2.5 trillion… or, in other words, 3.4% of global GDP at the time of research.
That is a ridiculous amount of money leaking out of the pockets of businesses across the globe. Money that, now more than ever, we simply cannot afford to waste.
Galvanic corrosion is arguably one of industry’s most pressing problems, but what is it, and how can we take the right preventative medicine?

Dangerous reaction
Galvanic corrosion is also known as dissimilar metal corrosion, which gives a fairly obvious clue as to why this electrochemical reaction takes place. When two dissimilar metals, such as steel and graphite, are coupled and exposed to a corrosive electrolyte (such as moisture) they corrode.
One of the metals becomes the anode in the reaction and corrodes faster than it would as a standalone material. The other (the nobler, with less chemical reactivity – for example, silver or platinum) becomes the cathode and corrodes slower than it would on its own. Gaskets containing graphite are a particular area of concern, as graphite is the noblest metal there is. This is important as the greater the difference in nobility between the metals, the greater the rate of galvanic corrosion.
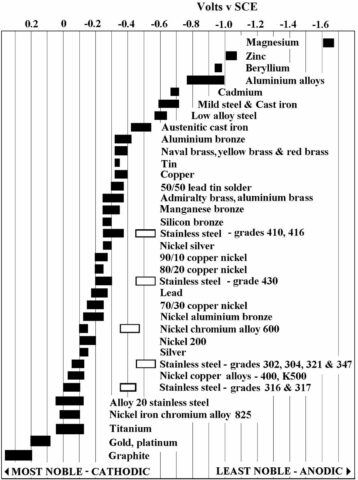
This degradation, with one of the materials undergoing a ‘supercharged’ process, undermines the integrity of the seal (or wider industrial infrastructure) and increases the likelihood of failure.
This is of particular concern in industrial segments such as hydrocarbon facilities, where leaks and failure can have catastrophic consequences, and maritime, with seawater acting as an all too effective electrolyte.
So, that’s the problem.
But what about prevention?
One of a kind
The solution is, frankly, right in front of our faces.
If dissimilar metals, such as those used in spiral wound gaskets, lead to dissimilar metal corrosion… then, well, don’t use dissimilar metals.
A solution such as Pipeotech’s DeltaV-Seal™ is the answer. The one-piece, CNC manufactured DeltaV-Seal is made from a compatible metal as the flanges it mates with. This means there is no electrochemical reaction, no anode and cathode, and no galvanic corrosion. And of course, no leaks.
The DeltaV-Seal is a ‘fit and forget’ solution. It has three unique sealing rings that deform on installation, filling any irregularities and creating the perfect seal, with no need for retightening or maintenance. No fire or blowout risk means no safety concerns and, with the problem of galvanic corrosion neatly sidestepped, no deterioration over the long-term, no matter how demanding the environment.
Make no mistake, galvanic corrosion should not be underestimated – but it can be avoided. And we can think of (at least) 2.5 trillion reasons why that is a good idea.
Give us a call, or drop the team a mail, to find out how we can help you prevent the problem of corrosion on your piping infrastructure… once and for all.
More from Case Studies
DeltaV-Seal - Reducing fugitive emissions in the Permian Basin
James Knights on Case Studies · Jul 22, 2024

The United States Clean Air Act (CAA) is the comprehensive federal law regulating emissions from stationary and mobile sources. Among other things the CAA authorises the United States Environment…
Best Available Technology to reduce ATEX zones, increase reliability, and improve safety within a refinery
James Knights on Case Studies · Jul 21, 2023

Europe’s most advanced refinery wanted to further pioneer by only utilizing Best Available Technology to ensure a safe and reliable operation with minimal impact on the environment, enhanced…
FPSO & Marine - DeltaV-Seals ensure the safe and reliable operation of inert gas systems
James Knights on Case Studies · Mar 21, 2023

A market-leading expert for the maritime industry manufactures a range of inert gas generators to suit their client's needs. Vessels carrying cargo-producing hydrocarbon vapors require inert gas…
Preventing nitrous gas leakage at a Thermowell instrument flange joint
James Knights on Case Studies · Mar 09, 2023

On a Thermowell instrument coupling flange joint a previous semi-metallic gasket required maintenance intervention 7 times in three-and-a-half years. Switching from a semi-metallic gasket to the…
Semi Conductor application for Nitrogen and Oxygen gas purifiers
James Knights on Case Studies · Feb 27, 2023
One of the world's leading semiconductor manufacturers in South Korea required a carbon-free, bacteria and virus-tight gasket capable of withstanding temperatures up to 500°C. The DeltaV-Seal™…
ATEX Zoning reduction & Equipotential bonding possible with the DeltaV-Seal
James Knights on Case Studies · Jan 09, 2023

Recycling metallic canisters containing flammable gases to prevent the emission of the gases to the environment and recycle the metallic packing material.
Reducing ATEX zoning within a biogas plant
James Knights on Case Studies · Nov 11, 2022

Local regulation of a biogas plant in Western Europe required ATEX zoning. Utilizing several technical standards including EN1127, EN1591, and Pipeotech's extensive library of product testing, it has…
Cleaned gaskets within a peroxide system of a chemical processing plant
James Knights on Case Studies · Oct 11, 2022
Ensuring product quality and plant safety with a clean, bespoke, tight, and durable seal. The DeltaV-Seal enabled a manufacturer of peroxide to ensure their product was made safely and was free of…
Corporate environmental responsibility ‘done right’ with long-term methane mitigation by design

Jo Shailes on Case Studies · Nov 02, 2021

Methane is one of the most potent greenhouse gases and avoiding leakage is a priority when using methane systems. Methane also ignites easily, so there are clear and immediate HSE incentives for…
Gasket ensures a long-lasting seal
James Knights on Case Studies · Nov 02, 2021

In manufacturing fertilizer, ammonium nitrate is added to the process, and traditional, spiral wound and Gylon gasket technology often deteriorate in these conditions, resulting in leaks. This is…
ATEX zoning unnecessary with DeltaV-Seal™

Jo Shailes on Case Studies · Nov 01, 2021

During final assembly in the factory, cars are filled with fuel before being transported to their final destination. To facilitate this, fuel lines run throughout the facility. However, along the…
DeltaV-Seal™ stops flammable leakage, prevent fires

Jo Shailes on Case Studies · Nov 01, 2021

In facilities that use hot oil heat transfer fluid systems, fire hazards are a significant challenge due to the combination of organic combustible liquids, which run at temperatures above their flash…
DeltaV-Seal™ prevent fires, promote safe environment

Jo Shailes on Case Studies · Nov 01, 2021

The use of hot oil systems in manufacturing presents challenges when traditional gaskets breakdown and cause leakage. As liquids in the system are kept at a temperature above their flash point, when…
DeltaV-Seal™ prevents leaks and pipe corrosion

Jo Shailes on Case Studies · Nov 01, 2021

In district heating and cooling, generic spiral wound gaskets frequently cannot cope with the cyclical temperature conditions and often fail, leading to leakages and corrosion of the pipeline. This…
DeltaV-Seal™ performs under extreme marine conditions

Jo Shailes on Case Studies · Nov 01, 2021

Importing ship-to-shore Liquefied Natural Gas (LNG) requires robust and efficient pipeline seals that are compliant with high environmental standards. The thermal cycling caused by the cryogenic…
DeltaV-Seal™ reduces greenhouse emissions

Jo Shailes on Case Studies · Nov 01, 2021

In fertilizer production, high-quality phosphorus fertilizers require nitric acid as a key ingredient, which is usually synthesized via the Ostwald process on-site (oxidizing of ammonia before…
In conversation with: Odd Skagestad, GE Healthcare Lindesnes
Geir Otto Amundsen on Case Studies · Jun 02, 2021

We recently spent some time talking with technical engineer Odd Skagestad from GE Healthcare Lindesnes about how DeltaV-Seal™ technology has impacted operations at a world-leading pharmaceutical…
Pipeotech’s reach goes atomic
Geir Otto Amundsen on Case Studies · Jan 05, 2021

Pipeotech has taken gasket tightness to new microscopic levels by supplying DeltaV-Seal™ to European Spallation Source, the world’s most powerful neutron source, in Lund, Sweden. The new research…
VARD finds the perfect fit

Jo Shailes on Case Studies · Nov 11, 2020

Norwegian-based shipbuilder VARD has been installing Pipeotech’s DeltaV-Seal™ in its vessels since 2016 and the cost-saving benefits have been very clear to Johny Morland, Principal Engineer, QA and…
Pipeline safety – How gasket choices can mitigate health and safety risks
Geir Otto Amundsen on Case Studies · Jan 31, 2020

Nothing is as important as health and safety when it comes to industrial facilities. However, a critical component in piping systems is often overlooked, viewed as a consumable rather than a key to…
Stopping expensive leakages at historic brewing facility
Geir Otto Amundsen on Case Studies · Jan 31, 2020

With the title of ‘the oldest brewery in Norway’, the Aass brewery is about as close as it gets to being a Norwegian institution. Situated on the banks of the river in the busy port city of Drammen…
DeltaV-Seal™ replaces kammprofile gaskets at critical distribution facility

Jo Shailes on Case Studies · Jun 12, 2019

After a long relationship utilizing kammprofile gasket technology, Norwegian LPG distributor PrimaGaz Norge AS has decided to abandon the old kammprofile gasket design in favor of the new DeltaV-Seal…
Flare tip leakage is too hot to handle for the competition
James Knights on Case Studies · May 15, 2019

Pipeotech was called upon to provide a solution for Flare Tip flange leak in the North Sea. A North Sea Operator came to Pipeotech with a problem affecting a Flare line on their platform. This was…
Exceeding gas-tight sealing strengths and eliminating ATEX zones
Geir Otto Amundsen on Case Studies · May 08, 2019

Gaskets seals are generally the consumables often selected at the last moment in a design project and carry few expectations. Although viewed as less significant, this consumable can have the most…
Intergas exclusively use DeltaV-Seal™

Jo Shailes on Case Studies · Mar 26, 2019

Following a highly successful test period, Intergas AS has announced that the DeltaV-Seal has been named as their exclusive seal of choice for all current and future liquefied natural gas operations.…


